The future of anesthesia care.

Transforming Anesthesia Care Through CO₂ Monitoring
A first-of-its-kind oxygen delivery and end-tidal CO₂ monitoring device designed for outpatient sedation, MAC1, TIVA2, and twilight anesthesia. The Aeris™ device improves patient safety, reduces costs, and saves critical time in ambulatory cases. It maximizes the utility of Guedel Oropharyngeal Airways and Nasopharyngeal Airways, airway management devices that currently lack supplemental oxygen delivery and CO₂ monitoring – transforming the hundred million plus of these devices used annually in the U.S. into advanced oxygen delivery and CO₂ monitoring systems – thereby improving the anesthesia providers ability to meet American Society of Anesthesiologists (ASA) guidelines3.
Overcoming Challenges in Outpatient Anesthesia
In outpatient anesthesia, effective oxygen delivery and CO₂ monitoring can be a struggle due to the use of “open systems” like nasal cannulas, oxygen masks, or even the jerry-rigged “tube and tape method4.” Unlike the “closed systems” in general anesthesia, these methods are less reliable.
The Aeris™ device modernizes outpatient anesthesia by providing targeted oxygen delivery and accurate real-time CO₂ monitoring. This ensures higher patient safety and meets the latest ASA guidelines.

Introducing the Aeris™
What the Experts Are Saying. . .
Revolutionary Features
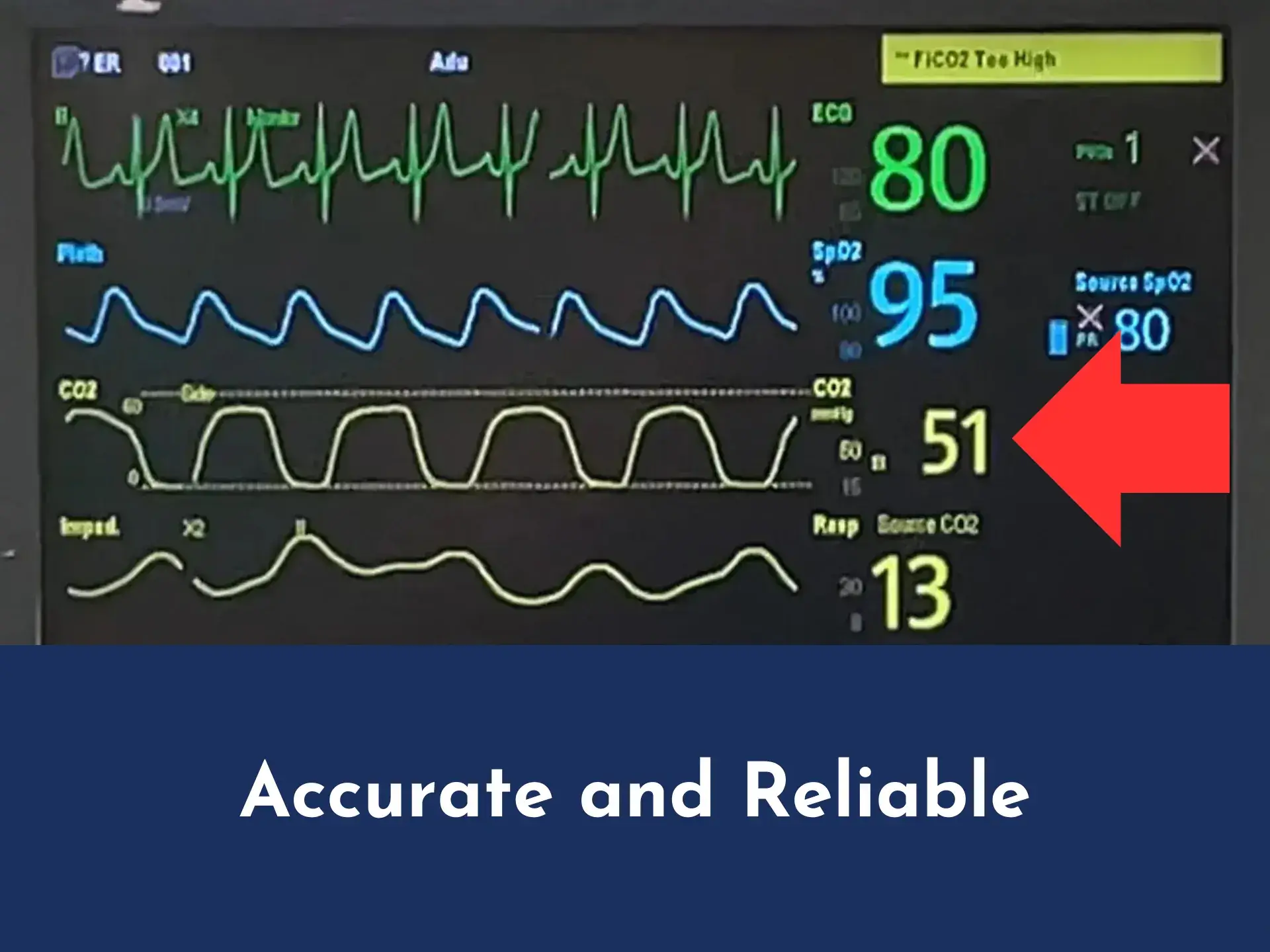
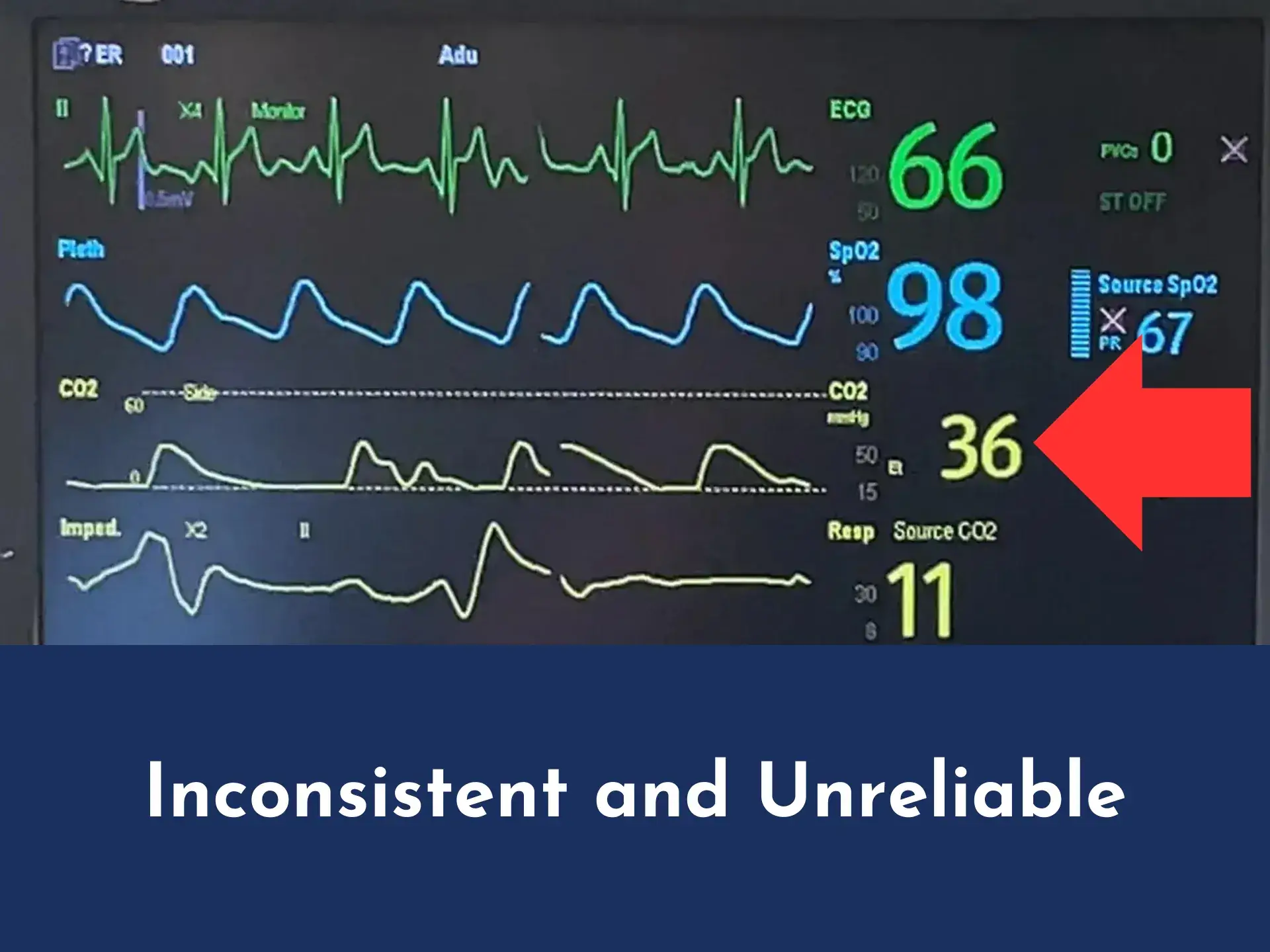
Enhanced CO₂ waveform via Aeris™ versus CO₂ waveform via Oxygen Mask with Capnography
Aeris™ capnography waveform
Oxygen Mask with Capnography waveform
Enhanced Oxygen Delivery
The Aeris™ delivers oxygen directly to the pharyngeal/supraglottic region, ensuring high-concentration oxygenation at flow rates as low as 2L/min.
Accurate Real-Time CO₂ Monitoring
The Aeris™ provides precise CO₂ readings and waveforms in real-time. This ensures that healthcare providers have the critical information needed to manage ventilation and sedation levels and respond quickly to patient needs.
Ease Of Use
The Aeris™ intuitively and seamlessly fits into all Guedel Oropharyngeal Airways and Nasopharyngeal Airways and can be used with all capnography monitors.
Improved Patient Safety
The Aeris™ improves patient safety by providing superior oxygen delivery and CO₂ monitoring during outpatient anesthesia. In addition, the Aeris™ minimizes fire risk seen when procedural oxygen masks or nasal cannulas are used. Instead of oxygen pooling around the patient’s head and neck, as occurs with masks and nasal cannulas, the Aeris™ targets oxygen delivery to the patient’s vocal cords, which allows for lower oxygen flow rates to achieve high FiO₂; thereby, minimizing the risk of a spontaneous flammable event.
Cost Savings and Workflow Efficiencies
Less oxygen will be required and a nasal cannula will not have to be cannibalized as is the case with the “tube and tape method”4 when the Aeris™ is used. By allowing the anesthesia provider to titrate sedatives and anesthetics using the capnometry and capnography information provided via the Aeris™, patients can be brought out of sedation at the optimal time to increase patient throughput.
No More Makeshift Solutions
With the Aeris™, healthcare providers no longer need to rely on antiquated devices like nasal cannulas or unreliable makeshift solutions like the “tube and tape” method to deliver oxygen and monitor CO₂. The Aeris™ offers a standardized, reliable approach to airway management by transforming Guedel Oropharyngeal Airways and Nasopharyngeal Airways into novel oxygen delivery and CO₂ monitoring systems.

FAQs
How does the Aeris™ device augment the utility of standard Guedel Oropharyngeal (OPA)⁵ and Nasopharyngeal (NPA) airways during procedural sedation?
The Aeris™ is a novel airway management device that seamlessly inserts into traditional Guedel OPAs and NPAs during procedural sedation to transform such airways into high-performing oxygen (O₂) delivery and end-tidal carbon dioxide (EtCO2) monitoring systems. By providing supplemental O₂ and continuously and reliably monitoring EtCO₂ in real-time, the Aeris™ reduces the risk of hypoxia and allows for early detection of respiratory compromise in sedated patients.
What is the quality of the EtCO₂ waveform produced by the Aeris™?
The CO2 intake port of the Aeris™ is strategically located at the central distal tip of the OPA or NPA, close to the hypopharynx – the optimal location to produce a continuous, reliable, real-time EtCO₂ waveform.
Will the Aeris™ work with existing capnometry hardware?
Yes, the Aeris™is compatible with pre-existing capnography monitors.
How does the use of the Aeris™ impact the workflow of the aesthesia provider?
Does the Aeris™ provide equal or better FiO₂ compared to a high-concentration rebreather bag/mask combination?
Not only does the Aeris™ device support FiO₂ levels equal to or better than facemask delivery systems, but the Aeris™ does so at low flow rates (~2L/min) compared to the rates required by masks (~8-15 L/min).
What procedural sedation cases would the Aeris™ provide the most benefit?
Due to the Aeris™ device’s optimal delivery of O₂ and monitoring of EtCO₂, the Aeris™ offers benefits to a wide array of medical procedures and surgeries where outpatient sedation, MAC, TIVA and twilight anesthetics are used. These include but are not limited to:
- Pain Management Cases
- Extremity Surgeries
- Faciomaxillary Surgeries
- Endoscopic Procedures
How does the AERIS™ perform during high O₂ flow rates?
At high O₂ flow rates, the Aeris™ maintains EtCO2 waveform fidelity, a feature not offered by EtCO₂-sampling nasal cannulas, procedural face masks, or the “tube and tape method.”4 This is accomplished by the Aeris™ having the O₂ delivery port and CO₂ intake port offset to minimize the dilution effect O₂ flow has on EtCO₂ detection.
The future is (almost) now!
The Aeris™ anticipates FDA-approval in Q3 of 2025.6
Stay Informed About the Future of Anesthesia Care
Be the first to know about the latest updates and availability of the Aeris™. Join our email list to receive exclusive information on our progress, launch dates, and sample availability.
Your privacy is of utmost importance to us. We won’t share your email address and will only contact you to send pertinent updates about our Aeris™ device. You can unsubscribe at any time.
What Drives Aeris™
The AERIS™ team consists of successful medical device innovators, esteemed clinicians and researchers, and highly regarded medical device sales and marketing executives. In totality, we have 65 years working in the medical device sector and 60 years of anesthesia care experience, hold 6 patents, have exited 8 medical device companies, and have been responsible for $580M in medical device sales. We are committed to using our experience and passion for medical device innovation and patient care to bring Aeris™ to outpatient clinics where moderate to deep sedation is used across the globe.
Thomas Kotoske, DO, FAOCO, FAACS
AERIS Co-founder & Board Member
Award Winning & Nationally Recognized Cosmetic Surgeon
Paul Fehrenbacher
AERIS CEO & Board Member
Chris Klecher
AERIS Board Member
Senior Vice President, Endoquest Robotics
Eric Wardrip, MD
AERIS Clinical Advisor
Medical Directior, SCA Center for Surgery of Encinitas
Cody Birch, CRNA
AERIS Co-founder & Board Member
Joe Voss
AERIS Director of Sales & Marketing
Stanley Zee
AERIS Board Member
Venture Capitalist, The IDEA Center at The University of Notre Dame
Thomas Kotoske, DO, FAOCO, FAACS
AERIS Co-founder & Board Member
Award Winning & Nationally Recognized Cosmetic Surgeon
Cody Birch, CRNA
AERIS Co-founder & Board Member
Paul Fehrenbacher
AERIS CEO & Board Member
Joe Voss
AERIS Director of Sales & Marketing
Chris Klecher
AERIS Board Member
Senior Vice President, Endoquest Robotics
Stanley Zee
AERIS Board Member
Venture Capitalist, The IDEA Center at The University of Notre Dame
Eric Wardrip, MD
AERIS Clinical Advisor
Medical Directior, SCA Center for Surgery of Encinitas

1MAC (Monitored Anesthesia Care) sedation is a type of anesthesia that provides pain relief and sedation during medial procedures while allowing the patient to remain conscious.
2Total intravenous anesthesia (TIVA) is a general anesthesia technique that uses a combination of intravenous anesthetic agents to induce and maintain anesthesia without inhalational agents.
3The American Society of Anesthesiologists (ASA) has specific guidelines for CO₂ monitoring, particularly during moderate and deep sedation. These guidelines were updated in 2010 and became effective in July 2011. During moderate or deep sedation, the adequacy of ventilation must be evaluated by continual observation of qualitative clinical signs and by monitoring for the presence of exhaled carbon dioxide.
4The “tube and tape method” refers to a common practice used during procedural sedation procedures, particularly in outpatient surgical settings, to monitor a patient’s EtCO₂ levels. A nasal cannula’s O₂ delivery CO₂ sampling tube are cut and taped to an OPA’s opening. This approach is considered a “jerry-rigged” solution, as it is not a standardized or optimal method. The main issues with this method are the tubes often slip out, especially if the patient needs to be moved, and it can provide poor quality EtCO₂ waveforms, resulting in minimal useful data.
5OPA = Guedel Oral Pharyngeal Airway, the most frequently used OPA
6The statements made regarding the Aeris™ device have not been evaluated by the Food and Drug Administration. FDA-approved research has not confirmed the Aers™ devices efficacy.